Sponsors
What is a teletrial?
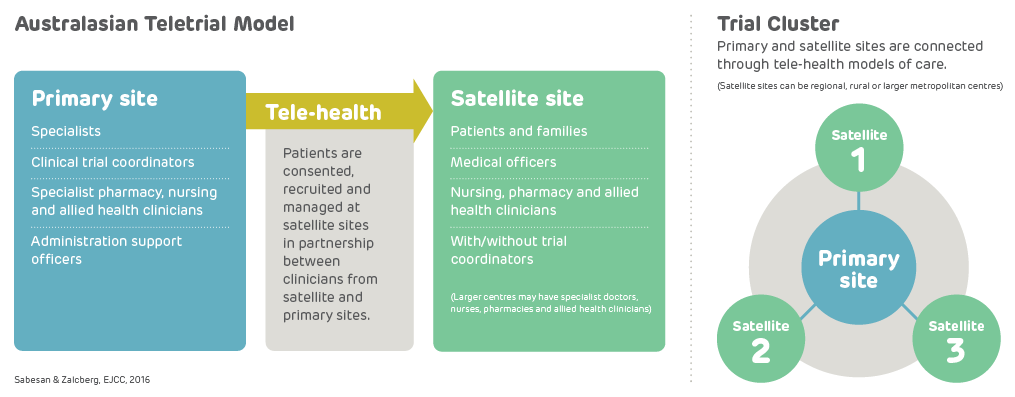
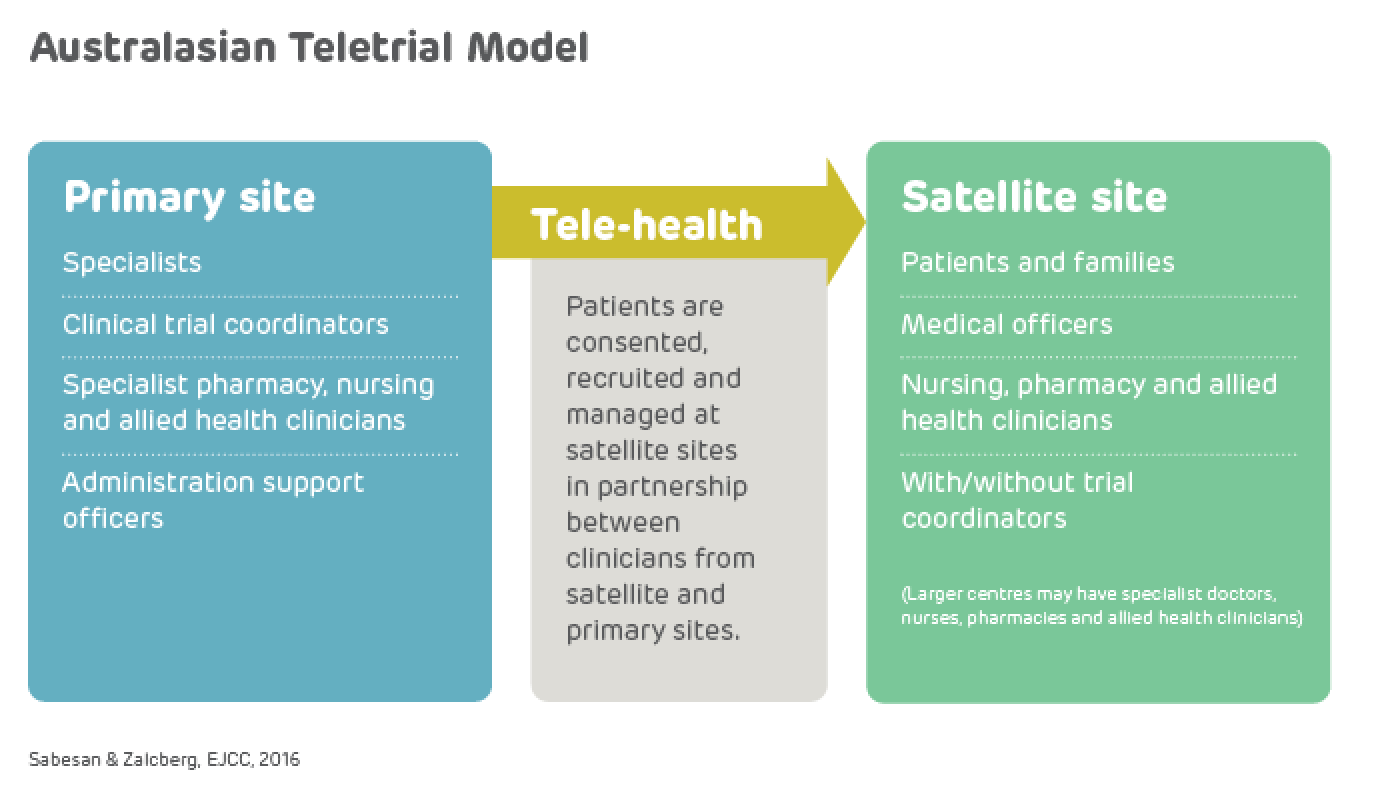
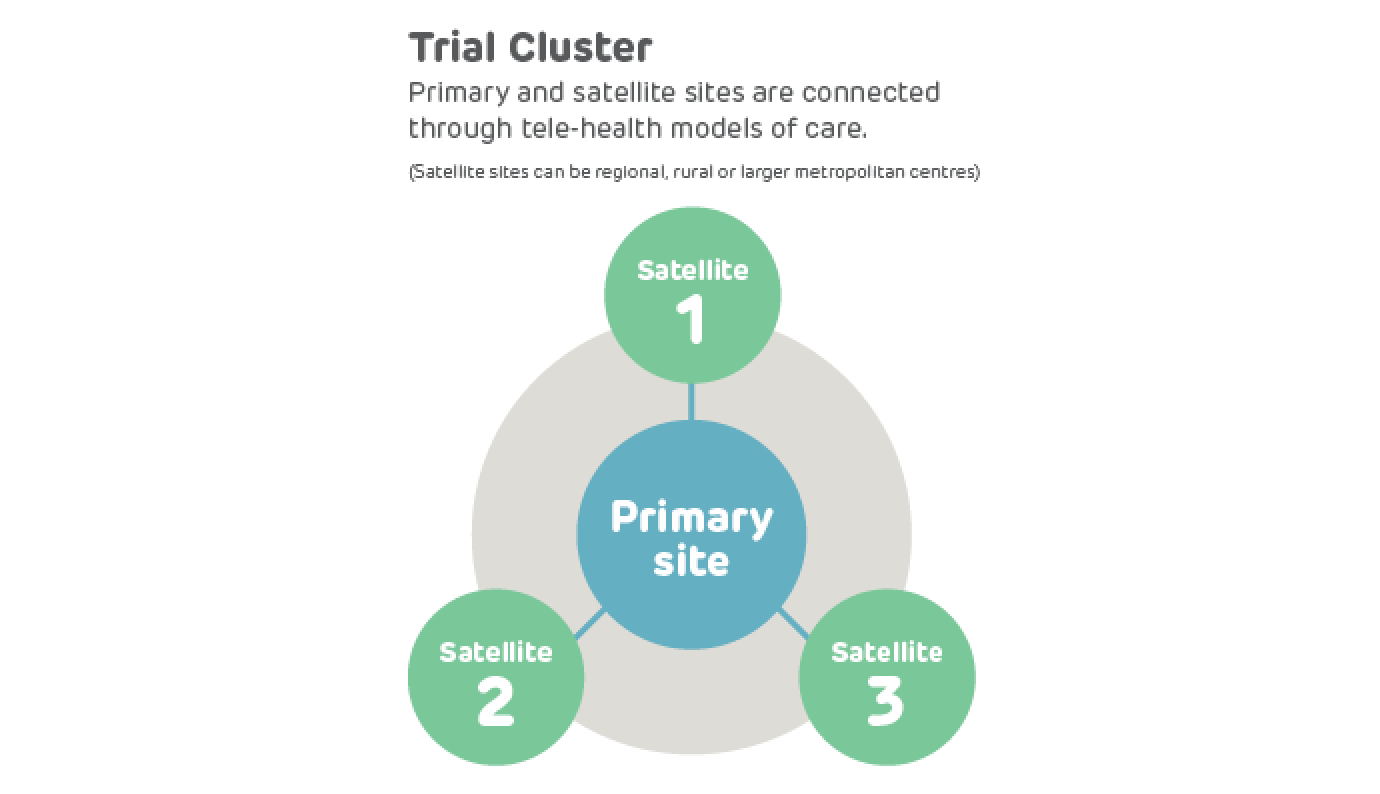
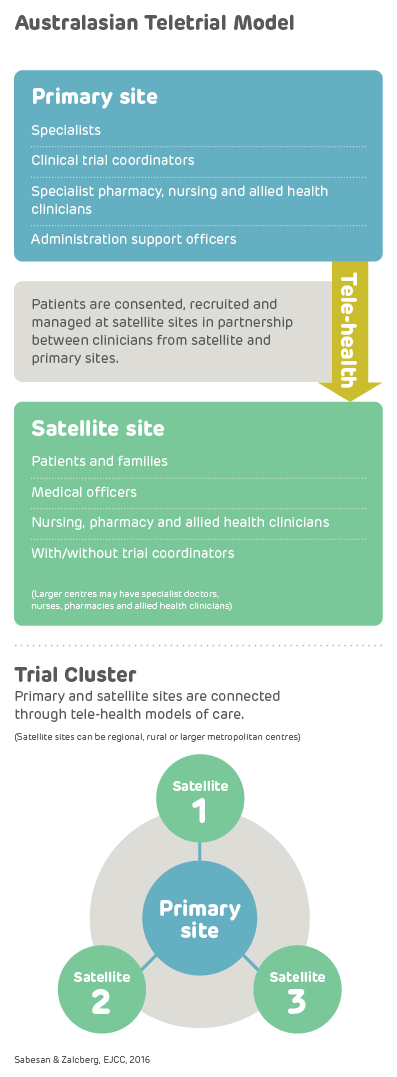
A teletrial is a group of clinical trial sites that work together to conduct a clinical trial under the supervision of a Primary Site.
A teletrial uses telecommunications technology to allow a Primary Site to work with a Satellite Site/s and deliver aspects of a clinical trial. This is outlined in a nationally harmonised Teletrial Supervision Plan and allows a Principal Investigator to supervise Associate Investigator/s to conduct clinical trials at a Satellite Site that is geographically remote from the Principal Investigator’s Primary Site.
The Principal Investigator remains responsible for the trial, and ICH GCP requires a Delegation Log for all Satellite Sites regardless of experience. Trial participants may have trial visits at both the Primary and Satellite Sites, as determined by the Protocol and Supervision Plan.
New Sponsor resources are available here. They were co-designed with the ATP Sponsor Advisory Group and underwent extensive consultation with several national stakeholder groups.
The conduct of the trial is detailed under the ‘head agreement’ (Clinical Trial Research Agreement/Clinical Trial Agreement) between the Sponsor and the Principal Investigator’s Institution and a Sub-Contract between the Primary Site and the Satellite Site Institutions (see Clinical Trial Research Agreement – Tele Trials Subcontract).

Case study
Global sponsor advocacy key for successful teletrial implementation across Australia
With decentralised trials known worldwide, General Manager of Research and Partnerships at Latrobe Regional Health Dr Jhodie Duncan says teletrials are the safety mechanism for patients within the Australian landscape.
Dr Duncan says teletrials not only allow access to care closer to home but provide the opportunity for local staff to develop their skills while managing the clinical trial under the supervision of a Primary Investigator. This saves patients travelling hundreds of kilometres and lets them stay in their care community for treatment, saves sponsors paying for travel, transport, and overnight stays and builds capacity and capability at regional, rural and remote services.
But Dr Duncan says we need more global sponsors to champion teletrials, because there is more education required to fully understand the benefits.
Dr Duncan cautions that in the current landscape, at least within Victoria, teletrials will not mean faster startups, less workload, or more significant financial gain. They do however offer greater recruitment and diversity. Two recent teletrials have had recruitment at the satellite site at or higher than the primary site. She says working with sponsors and CROs improves the teletrial model if they can work within site capabilities, are creative, adaptable and willing to learn.
Dr Duncan uses the example of a committed Sponsor who worked tirelessly to help their global team understand the benefits of adapting normal processes to deliver drugs direct to the satellite site, including the team joining late night calls to Europe.
Benefits
What are the benefits?

Recruitment & Retention
Potential for improvement in participant recruitment and retention, and increased diversity of participants in clinical trials

Harmonised Processes
National harmonisation of ethics and governance

Sponsors
How are teletrials different?
In implementing teletrials, jurisdictions will follow The National Principles for Teletrials in Australia. The National Principles have been developed to assist organisations engaged in conducting clinical trials in Australia to, wherever possible, standardise their procedures for key operations related to clinical trials and specifically teletrials.
How to get involved
Contact your local RCCC teletrials team:
Northern Territory
Email ATP-NT@nt.gov.au
Click here for information about Teletrials in the Northern Territory
Queensland
Email QRCCC@health.qld.gov.au
South Australia
Email ATP-SA@sa.gov.au
Click here for information about Teletrials in South Australia.
Tasmania
Victoria
Email RCCC@safercare.vic.gov.au
Click here for information about Teletrials in Victoria.
Western Australia
Email WARCCC@health.wa.gov.au
Get in touch
The Australian Teletrial Program (ATP) will improve access to, and participation in, clinical trials for rural, regional and remote Australians.
To receive ATP updates, sign up to our newsletter!





